Quantum Chemistry:
- What was the “plum pudding” model and who came up with it? JJ Thomson came up with the plum pudding model because he felt that the presently (in his time) accepted model of the indivisible atom did not take into account electrons (Thomson was the one who discovered electrons from his cathode ray tube experiment). His plum pudding model of the atom said that negatively charged electrons were stuck to a lump or mass of positively material.

- How was the plum pudding model lacking? This model discussed nothing of the number of protons/electrons; their arrangements in the atoms or the easiness with the atoms are stripped off their electrons (so they can form ions).
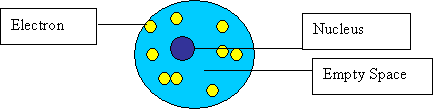
- What was the Rutherford model? Rutherford’s model of the atom was based off of his discovery of the nucleus (see Gold Foil experiment). He said that the atom had one dense nucleus surrounded by floating electrons and the rest was empty space.
- What the Bohr atomic model and in what year did its founder come up with that model? In 1913 Neil Bohr came up with the Bohr Model of the Planetary model. He said that electrons are configured in concentric circular paths called orbits around the nucleus. This model reflects the orbits that that planets take around the sun. He also explained that electrons in those fixed orbits have energy of their own, they do not lose energy and therefore do not fall into nucleus.
- What is an energy level? The energy level of an electron is the region around the nucleus where the electron is likely to be moving or orbiting. Energy levels can be compared to rungs of a ladder. The lowest energy level is the lowest rung of the ladder. Electrons cannot exist between energy levels. To move from one energy level to another, the electron must gain or lose just the right amount of energy.
- What is a quanta? It is the amount of energy required to move an electron from one energy level to the next higher one. Obviously, the more energy levels the electrons goes up the farther away it is from the nucleus.
- *** Important: The energy required to go up every energy level is not the same, as you get higher the distance between energy levels decreases, there between the higher energy levels, less energy is required to move up.
- What does an electron that is in the higher energy level escape from the atoms more easily than an electron that is in the lower energy levels? As the energy levels increase, the space between them decreases, therefore in the higher energy levels; less energy is required to move up.
- What is the quantum mechanical model for the atom and who came up with in what year? Erwin Schrodinger came up with this model in the year 1926. The quantum mechanical model is mostly a mathematical with few analogies to the physical world. This model like the Planetary model does restrict the energy of electrons to a certain but it does not so talk about an exact orbit that the electrons take around the nucleus. Instead, it estimates the probability of finding an electron in a certain position. **The book makes an analogy here of the model being like a “fuzzy cloud”, the cloud is more dense where the probability of finding an electron is high and less dense where it is low and even though it is not clear where the cloud ends, there is some probability of finding an electron a good distance away from the nucleus.
- Explain the system of orbitals in the quantum mechanical model (This is a badly written question, I realize that). This model assigns energy through the principle quantum number (n). Each principle quantum number refers to the major, or principle energy level. They are assigned values in order of increasing energy: n=1,2,3,4. Each principle energy level has sublevels within it. The first sub-energy level is called s, then p, then d, then f, then alphabetical order. The first energy level (n=1) has the sublevel 1s. The second energy level has the sublevels 2s and 2p. The third energy level has the sublevels 3s 3p and 3d (a note about the d orbital: even though it is considered to be in the third level, when writing electron configurations it is written along the fourth level because of it’s high energy if it was the 4d orbital it would be written after 5s level etc.) and so forth. The s sublevel has 1 orbital (each orbital has 2 electrons max.). The p sublevel has 3 orbitals the d sublevel has 5 orbitals and the f sublevel has 7 orbitals (odd numbers going up). Also each electron in one orbital has it’s own spin characterized by opposite spins like up down or -.5 and +.5 . If you are confused about this please refer to page 365 and 366 of the text book…it has good diagrams.
- What is the electron configuration? In a very vague sentence: The ways in which electrons are arranged around a nucleus in an atom.
- What is the aufbau principle? Electrons enter the atom at lowest energy level first.
- What is Pauli’s exclusion principle? An atomic orbital may at most have two electrons. Either or two electrons can occupy an orbital but never more and to occupy the same orbital the electrons must have opposite spins.
- What is Hund’s rule? When electrons occupy orbitals of the same energy level, one electron enters each orbital until they all (in that energy level) have one electron and then they start to pair up (remember orbital box diagrams?). **For an excellent table with elements, their orbital box diagrams and their electron configurations refer to the bottom of page 368 of text book.
Exceptions to electron configuration. Exceptions are there because atoms are most stable when their energy levels are completely empty or completely full, then they are most stable when they are half full, and not stable at all when they are partially full. So we promote the necessary electrons from another energy level so they are all stable. 5 exceptions you will be expected to know:
Cr
is [Ar]4s13d5. Notice that both 4s and 3d orbitals are half
full
Cu is [Ar]4s13d10.
Mo is[Kr]5s14d5.
Ag is [Kr]5s14d10.
Au is [Xe]6s14f145d10.
- How do you predict electron configuration for ions? Elements will gain or lose electrons to achieve configuration of a noble gas. Na: 1s22s22p63s1. The closest noble gas neon, so sodium will want to stabilize itself to get neon’s configuration. It will do this by giving up one electron because it is easier to do that than gain seven. Therefore the predicted electron configuration for the Sodium Ion is Na+: 1s22s22p6
- **Light is a wave phenomenon**
- What is electromagnetic radiation? They are a series of energy waves that travel in a vacuum at the speed of 3.0 X 108 m/second; they include, infrared waves, microwaves, radio waves, ultraviolet waves visible light, x-rays and gamma rays.
- What is the amplitude of a wave? It is the wave’s height from the origin to the crest.
- What is the wavelength? It is the distance between the crests in a wave. It is represented by the Greek letter lambda (λ).
- What is the frequency of a wave? The number of wave cycles to pass a given point in a certain unit of time. It is represented by the Greek symbol nu (ν).
- What is the relationship between wavelength and frequency? Wavelength and frequency are inversely related. Also wavelength times frequency equals the speed of light: c=λν
![[The Electromagnetic Spectrum]](./Quantum%20Chemistry_chapter13_files/image004.gif) This is
a diagram of the electromagnetic spectrum.
This is
a diagram of the electromagnetic spectrum.
- What are units of frequency? The SI units are HERTZ (Hz). It can also be reciprocal seconds s-1 or one over s.
- What is the visible spectrum? In the electromagnetic spectrum it is the part where the light travels at the frequency around 3 X 1014. The visible spectrum ranges from red which is of lower frequency to violet, which is of a higher frequency. The other parts of the electromagnetic have frequencies either too high or too low to be seen by the human eye. (for another diagram refer to page 373 in text book).
- When do elements emit light? When they are excited by an electric discharge through it’s vapor or gas (cathode ray tube connection). The atom first absorbs the energy and then immediately looses it causing light emission.
- What is the atomic emission spectra? When one passes the light emitted by a single type of element through a prism one gets that atom’s atomic emission spectra. The atomic spectra consist of relatively few lines. This is known as line spectra or a discontinuous spectra. Each line of the spectra corresponds to one exact frequency of light emitted by the atom. Therefore each line corresponds to a specific amount of energy being emitted.
- What is ground state? Lowest energy level occupied by an electron when an atom is in its most stable energy state.
- What is Planck’s constant? Plank’s equation shows mathematically that amount of radiant energy absorbed a body is proportionate to the frequency of the radiation. Planck’s constant h which equals 6.6262 X 10-34 J X S. J is joule the SI unite for energy. E=h X ν. Energy of a quantum equals Planck’s constant times frequency and any attempt to raise in increments of less than h X ν will fail.
- How does an electron move up from ground state? If it gains a quanta of energy.
- How does atomic emission occur? Electrons in a atom absorb quanta of energy and move up from their ground state (this is called absorption). Now the atom is very agitated (excited). Immediately after this the atom emits the same amount of energy in the form of photons when the electron is dropped from the excited state to the ground state. Only electrons in transition from high energy level to lower ones emit light (this is called emission).
- What is the Lyman series? When very excited electrons drop to the first energy level. This produces ultraviolet light.
- What is the Balmer series? When highly excited electrons drop to the second energy level. This emits visible light.
- What is the Paschen series? When excited electrons drop to the third energy level. This emits infrared light.